Deep Analysis of TiO2 Nanoparticles
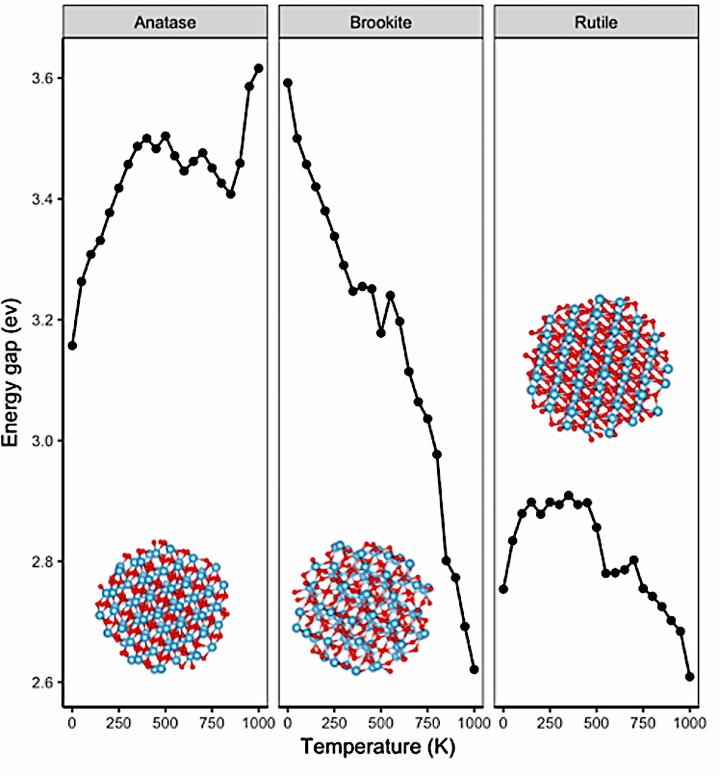
In this study, we perform a theoretical investigation using the density functional tight binding (DFTB) approach for the structural analysis and electronic structure of anatase, brookite and rutile phase TiO2 nanoparticles (NPs). Our results show that the number of Ti-O bonds is more than that of O-O while the number of Ti-Ti bonds are the less. Thus, large amount of O atoms prefers to connect to Ti atoms. The increase in the temperature of the NPs contributes to an increase in the interaction of Ti–O bonding, but a decrease in the O-O bonding. The segregation of Ti and O atoms shows that Ti atoms tend to co-locate at the center, while O atoms tend to reside on the surface. Increasing temperature causes decrease of the bandgap from 3.59 to 2.62 eV for brookite phase, which is much more energetically favorable compared to bulk while it could increase the bandgap from 3.15 to 3.61 eV for anatase phase. For three phase TiO2 NPs, LUMO and Fermi levels decrease. The HOMO level of anatase phase NP decrease, but it increases for brookite and rutile phase TiO2 nanoparticles. Herein, an increase in the temperature contributes to the stabilization of anatase phase TiO2 NP due to decrease in the HOMO energies.